Blog
How can you efficiently manage your products on the NHS dm+d database?
Dec 2, 2022
As a Pharma company, managing product information on the NHS dm+d database is essential to ensuring your medicines are prescribed to patients.
Datapharm held a live training session on how to efficiently submit and update product information on the NHS dm+d database. The webinar included an introduction to the history of the NHS dm+d and how the Electronic Prescription Service (EPS) works. Watch Now
What is the NHS dm+d?
The NHS dm+d (dictionary of medicines and devices) is a database which holds product information about medicines being prescribed and dispensed by UK healthcare professionals via the EPS.
It was originally developed after the NHS identified the need to standardise the way in which medicines and devices were described across NHS IT systems. This meant that one IT system in a particular care setting (such as a Pharmacy) could then communicate with another system in a separate care setting.
Submitting products to the NHS dm+d database
As a Pharma company, putting your product information on the dm+d database while keeping it up-to-date is essential to having your medicines prescribed to patients.
It can be a considerable amount of work going through the submission process or updating information for multiple products. Furthermore, product information such as pricing can change at any time which sometimes means updating multiple items at once.
So, could this work be simplified by using an effective and trusted tool?
Our dm+d submissions tool is a submission platform for the NHS dm+d database supporting over 150 Pharma companies, and is part of the
The dm+d submissions platform is the companion tool for the emc med data browser. These tools have been designed to provide simple, quicker submissions and smarter searches of important product information, including price, stock information, product codes, strength and route of administration.
The solution continues to be supported by updates and enhancements, as well as a dedicated Customer Success team to answer queries and provide training.
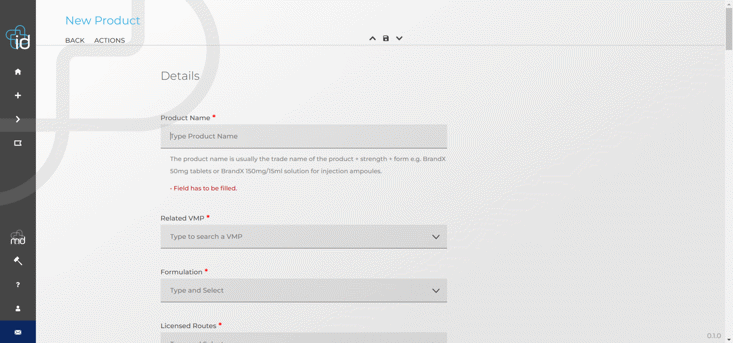
Live training webinar: How to efficiently manage your products on the NHS dm+d database
On Tuesday 6 December, Datapharm held a live training webinar on the emc med data (dm+d) tool to share best practice tips and support your work with submitting product information.
Key takeaways:
- The background of the NHS dm+d database, and how the electronic prescribing system works
- How to efficiently submit product information in as few clicks as possible
- How to manage and update product information on the dm+d database
- The tracking capabilities of emc med data (dm+d)
- Have your questions answered by our Customer Success team during our live Q&A
Watch the recording of our training session, here.
Have questions about submitting products on the NHS dm+d database?
If you’re a Pharma company looking at how to manage your product information on the dm+d database, get in touch with our team.