Pharma
View your own and competitor product information in the dm+d database.
Pharma needs to make its product information available to a wide variety of stakeholders for market access, regulatory, commercial and logistical purposes.
emc market access includes the med data suite, supporting Pharma with sharing and managing product information.


View your own and competitor product information in the dm+d database.

Search product information, availability and pricing.

Integration of content into prescribing and dispensing systems.

Electronic Prescription Services (EPS), interoperability and reimbursement.
It is essential to list your products on the dm+d database to ensure they are prescribed and dispensed by the NHS via the Electronic Prescription Service (EPS).
The dm+d submission tool is trusted by leading organisations for submitting and updating products to this database, as well as offering audit & tracking capabilities.
It is crucial to share product information with stakeholders such as wholesalers and the NHS, but also to do this safely and efficiently.
emc market access's supply chain platform streamlines your internal processes, enabling cross-team collaboration and a sustainable way to manage this information throughout the supply chain.
The supply chain platform also ensures you have a safe way to share this information, minimising the cyber security risks which come with sharing information over channels such as email.

Pharma needs to understand and look up the NHS dm+d database to ensure products are listed correctly and also to gain competitor intelligence, such as changes in pricing. It is also important to use a single source of truth for sharing comprehensive product information with other stakeholders throughout the supply chain.
The emc market access solution (emc med data) includes an intuitive browser to simplify these tasks and track products or suppliers of interest on the dm+d database and emc supply chain database.
We devote ourselves to providing you with a high-standard service so that you can take full advantage of the dedicated software.
All emc market access customers are given full support from our Customer Success team, via phone, video call or email. Working with us means making your product information available to HCPs or other relevant stakeholders without any unnecessary hurdles in your work.

The dm+d submission tool, included in emc market access, ensures timely submission of your product information to the NHS dictionary of medicines and devices (dm+d) and empowers Pharma to make sure their product information is available for prescribing as soon as possible. It is the most efficient method for submitting new and updated product information.
The supply chain submission platform provides Pharma with a Product Information Management (PIM) solution for a single source of truth for all product information requirements. It allows Pharma to centralise, manage, standardise, enrich, and efficiently distribute its product information to important internal and external stakeholders.
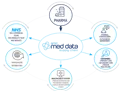
When creating a new product pack in the supply chain platform, you can input up to 70 attributes per pack, such as industry-specific codes, storage information and product pricing:
Pharma enters product pack information into the submission tool
The platform enriches and standardises product information, including data from emc and the dm+d databases
Stakeholders access the product information via emc med data, an automated API or Pharma can extract a file and send via email
The browser component of emc market access gives users access to the dm+d database, allowing a fast and effective way to check product price, availability, medicine, and devices information. Users can also access Pharma product information submitted through the supply chain platform.
A training webinar on how to use the emc market access solution's browser tool. Our Customer Success team show you how to efficiently look up products on the NHS dm+d database to view vital logistical information, track your competitors' products and refine your housekeeping.

The emc med data platform is very intuitive to navigate. It makes our lives easy, prompting us on which steps to take when we're working with new changes in product information such as discontinuations."
- Roche
The NHS Business Services Authority (NHS BSA) receives new data to the dm+d database on a daily basis. Once a submission has been actioned by NHS BSA, there can be a delay of up to 5 working days before it can be viewed on the emc market access platform. Allow a minimum of 2 weeks between submission and the day on which the update will be effective.
Each product submission to the dm+d database needs to be done individually as this is the preferred way for NHS BSA to receive the requests. However, you can quickly update product prices using the Mass Price Update functionality in emc market access. This allows changes to more than one pack price at the same time.
Yes, if you know your launch date in advance, you are able to submit your product information up to 3 months prior, allowing for early review by NHS BSA and provision of AMPP codes.
Yes, our intuitive, one-page submission tool enables you to submit your product information to NHS BSA via an online platform, allowing you to collate and track your changes, and keeping you in control of your product information.
BNF requires the medicine to be in the dm+d database prior to submitting information to them, in order to ensure that accurate prices are reflected in the BNF.
Any pharmaceutical stakeholders can access the supply chain database using the emc market access portal. Please register here to create an account on emc med data and our Client Services team will review your request.
As often as your product details change. The product information will be used by your stakeholders for a variety of purposes including ordering, fulfilling orders, and updating their IT Systems.
Previously, emc in-demand was provided as a stand-alone submission tool for product information into the ‘dm+d’, it is now offered as part of the ‘emc market access’ solution, which supports Pharma with:

Phone: +44 1372 371444
Email: [email protected]
Address:
Cassini Court, Randalls Way, Leatherhead, Surrey, KT22 7TW, United Kingdom