Blog
What is structured data and unstructured data in a Healthcare context?
Dec 6, 2022
Using structured data in Healthcare ultimately this means improved patient safety through better decision-making about medicines.


Simon Zedlewski
Content Manager, Datapharm
The following article is credited to Arif Govani. It is based on expert discussions during the webinar, ‘The future of electronic Product Information (ePI)’.
Using structured data with medicines information enables HCPs (healthcare professionals) and patients to be made aware of important information about a medicine which may affect the specific patient. Ultimately, this means improved patient safety through better decision-making about medicines.
But, in the context of Healthcare, what exactly does structured data mean?
What is structured data?
As mentioned in one of our previous articles, structured data means organising data into specific fields as part of a common layout, so having data structured means that there is an element of control over what goes where in the product information. Each field has a defined purpose so that:
- It can be easily looked up
- It is easy to access
- It is shareable
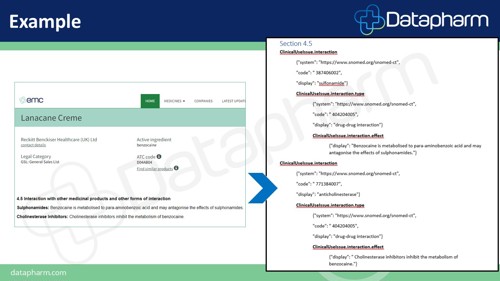
The above example of a section 4.5 within the SmPC (Summary of Product Characteristics) shows how product information is organised into specific fields, e.g. ‘ClinicalUseIssue’ is an example of a data structure. This particular field is used to highlight interactions with other medicinal products which could affect the patient.
What is unstructured and semi-structured data?
Conversely, product information can be completely unstructured. Consider a PDF – because this data is unstructured (i.e. text and images are fixed in place), IT systems like those used in Healthcare can’t easily interpret it or exchange the information.

The above example of a Patient Information Leaflet (PIL) in PDF format shows information in text form – it is fixed and cannot be tailored to another format, extracted or effectively linked to Healthcare systems.
Currently on emc, product information in SmPCs and ePILs (electronic Patient Information Leaflets) is in a semi-structured format. This means that it contains some structured elements (e.g. set headings and controlled vocabularies for active ingredients which are tied to the NHS dm+d database and SNOMED-CT), and some unstructured elements (e.g. free text and graphics).
How does emc support Pharma and Healthcare with structured data?
Medicines data on emc is updated in real-time and made instantly available to our established audience of over 80 million Pharma, HCPs, patients and other healthcare industry professionals.
We currently convert PILs (Patient Information Leaflets in pdf form) into ePILs (electronic Patient Information Leaflets) as part of our regulatory solution, enabling patients and prescribers to have increased visibility on vital medicines information in a user-friendly, semi-structured format.
Looking onwards into 2023, we have been working with Gravitate Health and HL7 as part of their Vulcan Accelerator programme to develop a worldwide data standard shaping how we use structured data in Healthcare. During the project, we looked at how we currently structure the data and what the future roadmap looks like to make the information more accessible for the Healthcare ecosystem. The new data standard is up for ballot in January 2023.
Learn more about how Healthcare can benefit from structured data
By structuring medicinal product information, we can positively impact patient safety by linking it to the patient record. Read our article on what structured data could do for healthcare to find out more: