Blog
How sophisticated is your electronic product information (ePI)? Benchmark against our Maturity Model
May 31, 2022
Datapharm has created an ePI Maturity Model, to help organisations easily and clearly identify their current level and understand the key steps that will help them advance to the next.

Karina Gomez
Marketing Manager, Datapharm
The widespread implementation of electronic product information (ePI) in the healthcare industry has the potential to significantly decrease human error and enhance patient adherence to safety information.
Furthermore, ePI that is connected, coded, and structured supports interoperability and greater data intelligence.
Datapharm has been providing electronic product information that is used all round the world for over 20 years. Datapharm holds structured medicines information on emc, aligned with the EMA’s proposed ePI format, and has been assisting over 400 Pharma customers in their digitalisation journey.
“The digitalisation journey is different for every pharma organisation that we speak to, as such, Pharma companies sometimes struggle to understand how to move forward and what ePI implementation might mean for their organisation. Therefore, we have created an ePI Maturity Model, which we hope will help organisations easily and clearly identify their current level and understand the key steps that will help them advance to the next”, said Chief Digital Officer, Arif Govani.
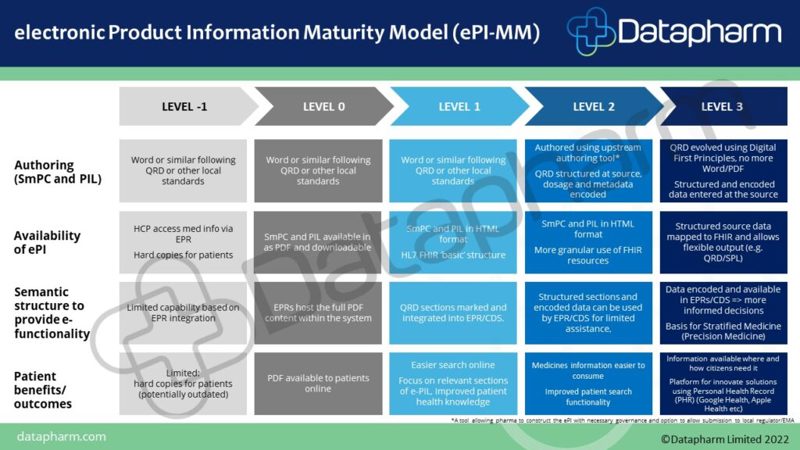
ePI Maturity Model - Contact [email protected] to request the full document
Interested in learning more about the status and future of ePI?
Watch this webinar where a panel of experts share best practice advice for greater adherence of patient safety information. Speakers include:
- Arif Govani, Chief Digital Officer, Datapharm,
- Craig Anderson, Director, Information Management, Pfizer,
- Martin Ingvar, Barbro and Bernard Osher Professor of Integrative Medicine, Karolinska Institutet
- Dr. F.A. (Fakhredin) Sayed Tabatabaei, Project Coordinator ePI (electronic Product Information), Medicines Evaluation Board
Key takeaways:
- How Pharma companies are making the transition to digital, with real-life data
- How regulation supports digital information and what needs to change
- Quick wins for Pharma to increase and measure engagement with its patient safety information
Get in touch with our team to find out how we can help you make your medicines information more readily available to both internal and external stakeholders, meeting regulatory requirements and in a user-friendly format.
Contact our team today.
