Blog
Datapharm pairs up with MILE to provide self-service SRDs
Feb 28, 2023
Datapharm and MILE are excited to announce the launch of a new SRD pilot, enabling HCPs with greater access to vital medicines information.

Datapharm is excited to announce the launch of a new Scientific Response Document (SRD) pilot, in collaboration with MILE (Medical Information Leaders in Europe).
Bringing the SRD pilot to emc means this will be the first time in the UK that SRDs from multiple pharmaceutical companies will be available in the same trusted and highly visited location.
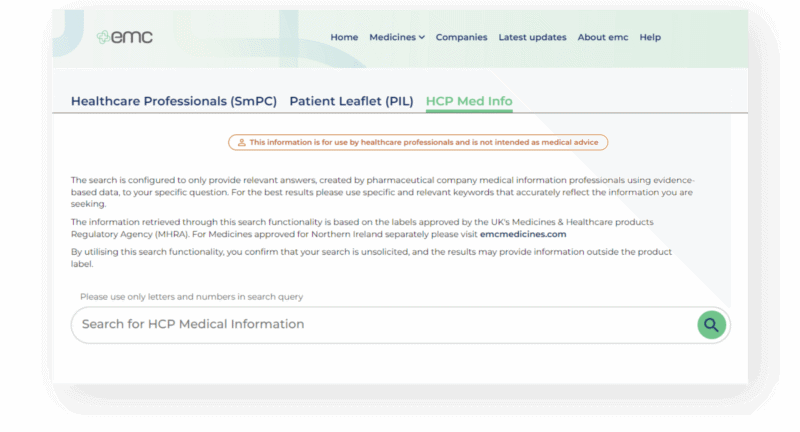
What are SRDs?
Scientific Response Documents (SRDs) in healthcare are written documents designed to answer frequently asked questions about specific medicines. They can often answer questions that are not directly covered by the SmPC (Summary of Product Characteristics) or other frequently published medicines information resources.
It is important for scientific response documents to be accurate, concise, and well-organised, as they may aid important clinical decisions.
What is the SRD Pilot?
The SRD pilot is an innovative cross-industry collaboration that brings together four major pharmaceutical companies, as well as Datapharm and MILE, to pilot a new service for UK Healthcare professionals (HCPs).
When visiting the emc website, UK HCPs will have an additional feature available on a select number of prescription medicines which are included in the pilot. The new feature enables them to search for information within SRDs for these medicines.
Speaking on the launch of the pilot, Datapharm’s CEO, Rich Cooper, said: “We're thrilled to be collaborating with MILE on this pilot, as our involvement in the project aligns perfectly with our devotion to improving the accessibility, effectiveness and excellence of healthcare product information. emc users have shown a great amount of interest in accessing self-service SRDs and, as a website which receives over 7 million visits per month, emc is an ideal stage for the Pharma industry to put itself in front of UK HCPs.”
Jill Voss, President of MILE, also spoke of her delight in the collaboration: “Five years ago MILE had a vision to improve access to the wealth of accurate and balanced information prepared by Medical Information professionals to answer specific unsolicited requests. We embarked on a journey to create a platform to enable HCPs to self-serve and satisfy their need for knowledge, when they want it and where they want it. We are so proud that our collaboration with Datapharm is a critical advancement in our mission. This is a unique and innovative pilot that brings essential information to clinicians at the point of need.”
Supporting HCPs’ access to important content on medicines
The pilot provides an invaluable opportunity to validate the value for HCPs having access to self-service SRDs, while proving there is engagement and support from the Pharma industry. By enabling Medical Information teams to make this content available to HCPs, those advising patients on medicines can then access important information on-demand at their time of need.
Watch a guided tour of the SRD pilot on emc
Datapharm hosted a live webinar to guide HCPs through the new functionality and demonstrate how this feature will be used. This webinar is now available on-demand.
The SRD pilot officially launches on 15th February 2023 and will run for 6 months.
About Datapharm:
Datapharm is the leading medicines information provider in the UK. Its medicines information database, emc (electronic medicines compendium) is the UK’s most comprehensive, trusted and accessible source of information on medicines, listing more than 10,000 medicines and attracting a growing audience of over 84 million visitors per year.
As a SaaS company, Datapharm provides innovative solutions to support the Life Sciences and wider Healthcare sectors in their work with medicines information, serving more than 350 Pharma customers in the UK.
About MILE:
Medical Information Leaders in Europe (MILE) started in 2014 as an informal gathering of Medical Information leaders and quickly grew in size and influence. Today it has 28 members that collectively represent a wide range of pharmaceutical companies across the region.
Working across Europe, MILE is dedicated to the provision of accessible, accurate and balanced medical information that supports the work of healthcare professionals and the patients they serve. As an industry association, it brings together leaders committed to the creation of innovative medical information solutions that reflect evolving trends in this crucial area of the pharmaceuticals business.